This comprehensive guide will provide you with all the information you need about ISO 13485:2016, a globally recognized standard for medical device quality management systems. We will delve into the core principles, key requirements, and benefits of implementing this standard. You’ll also find a free download link for the ISO 13485:2016 PDF, allowing you to access the official document and start your journey towards enhanced medical device quality.
What is ISO 13485:2016?
ISO 13485:2016 is an internationally recognized standard that specifies requirements for a quality management system (QMS) for organizations involved in the design, development, production, and distribution of medical devices. Its purpose is to ensure that medical devices are safe, effective, and meet the needs of patients and healthcare professionals.
Key Requirements of ISO 13485:2016
The standard outlines several key requirements that organizations must meet to achieve compliance. Some of the most important aspects include:
- Management Responsibility: Establishing a clear management structure and defining responsibilities for quality management.
- Documentation: Implementing documented procedures and records for various aspects of the QMS.
- Resource Management: Ensuring adequate resources, including human resources, facilities, and infrastructure.
- Product Realization: Establishing a controlled process for the design, development, production, and distribution of medical devices.
- Measurement and Analysis: Implementing processes for monitoring, measuring, and analyzing the effectiveness of the QMS.
- Nonconformance and Corrective Action: Establishing procedures for identifying and addressing nonconformities and implementing corrective actions.
- Customer Satisfaction: Ensuring that customer needs and expectations are met.
Benefits of Implementing ISO 13485:2016
Adopting ISO 13485:2016 can bring numerous benefits to medical device manufacturers, including:
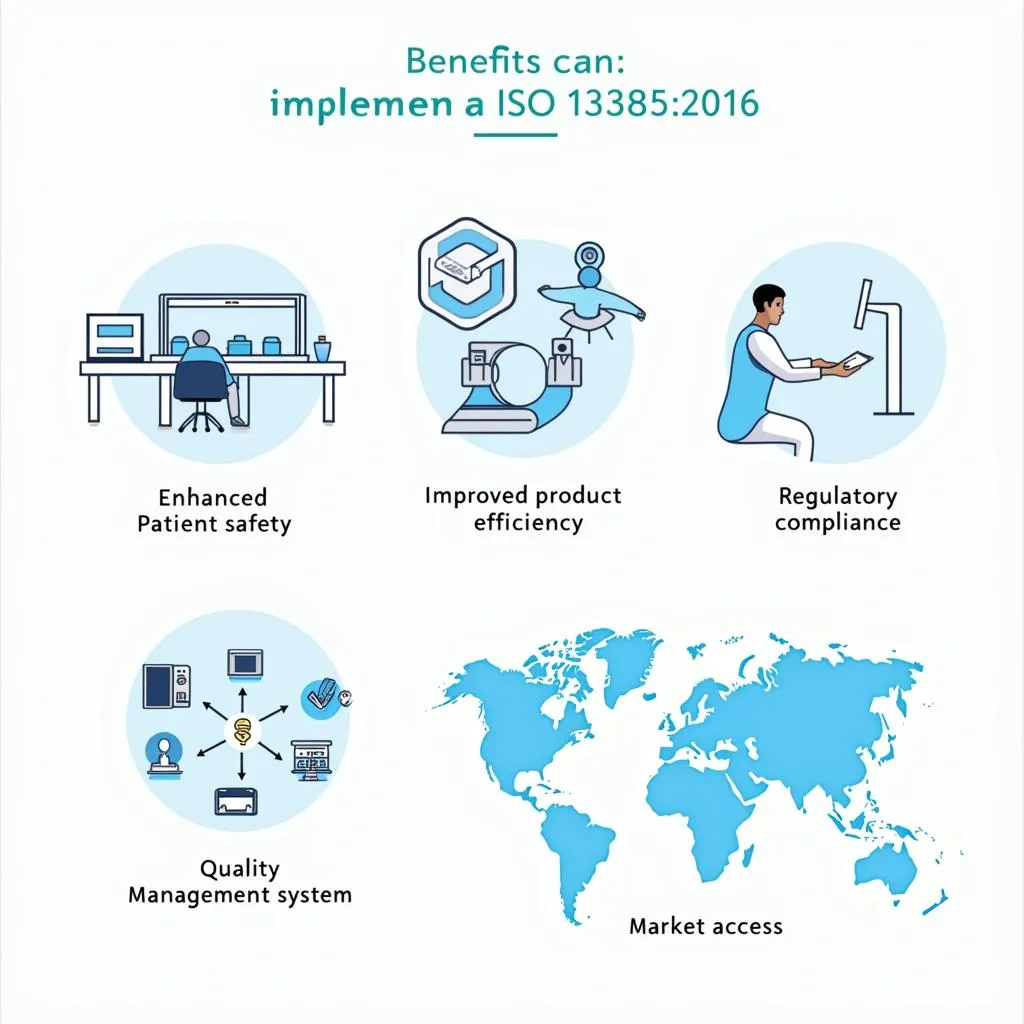
- Enhanced Patient Safety: By implementing a robust QMS, organizations can significantly reduce the risk of defects and ensure the safety of medical devices.
- Improved Product Quality: ISO 13485:2016 fosters a culture of quality throughout the organization, leading to higher product quality and fewer defects.
- Increased Efficiency and Productivity: Streamlined processes and documented procedures enhance operational efficiency and productivity.
- Enhanced Regulatory Compliance: Compliance with ISO 13485:2016 demonstrates commitment to quality and simplifies regulatory audits.
 Benefits of ISO 13485:2016 Implementation
Benefits of ISO 13485:2016 Implementation
- Enhanced Market Access: ISO 13485:2016 is recognized globally, opening up new markets and opportunities for manufacturers.
“ISO 13485:2016 is a vital tool for any organization involved in the medical device industry. It provides a comprehensive framework for ensuring the quality, safety, and effectiveness of medical devices, ultimately contributing to patient well-being.” – Dr. Emily Carter, Medical Device Quality Expert
Where to Download the ISO 13485:2016 PDF for Free
While the official ISO 13485:2016 document is not available for free download, several reliable sources offer the standard at a reasonable cost. These sources include:
- ISO Website: The official website of the International Organization for Standardization (ISO) offers the standard for purchase.
- National Standardization Bodies: National standardization bodies, such as the American National Standards Institute (ANSI) and the British Standards Institution (BSI), also offer the standard.
- Online Retailers: Several online retailers, including Amazon and Barnes & Noble, offer the ISO 13485:2016 standard in PDF format.
“The ISO 13485:2016 PDF provides a clear and detailed roadmap for implementing a robust quality management system. It’s an invaluable resource for organizations striving for excellence in medical device production.” – Mr. John Davies, Medical Device Regulatory Affairs Specialist
Implementing ISO 13485:2016
Implementing ISO 13485:2016 requires a structured approach. Here are some key steps:
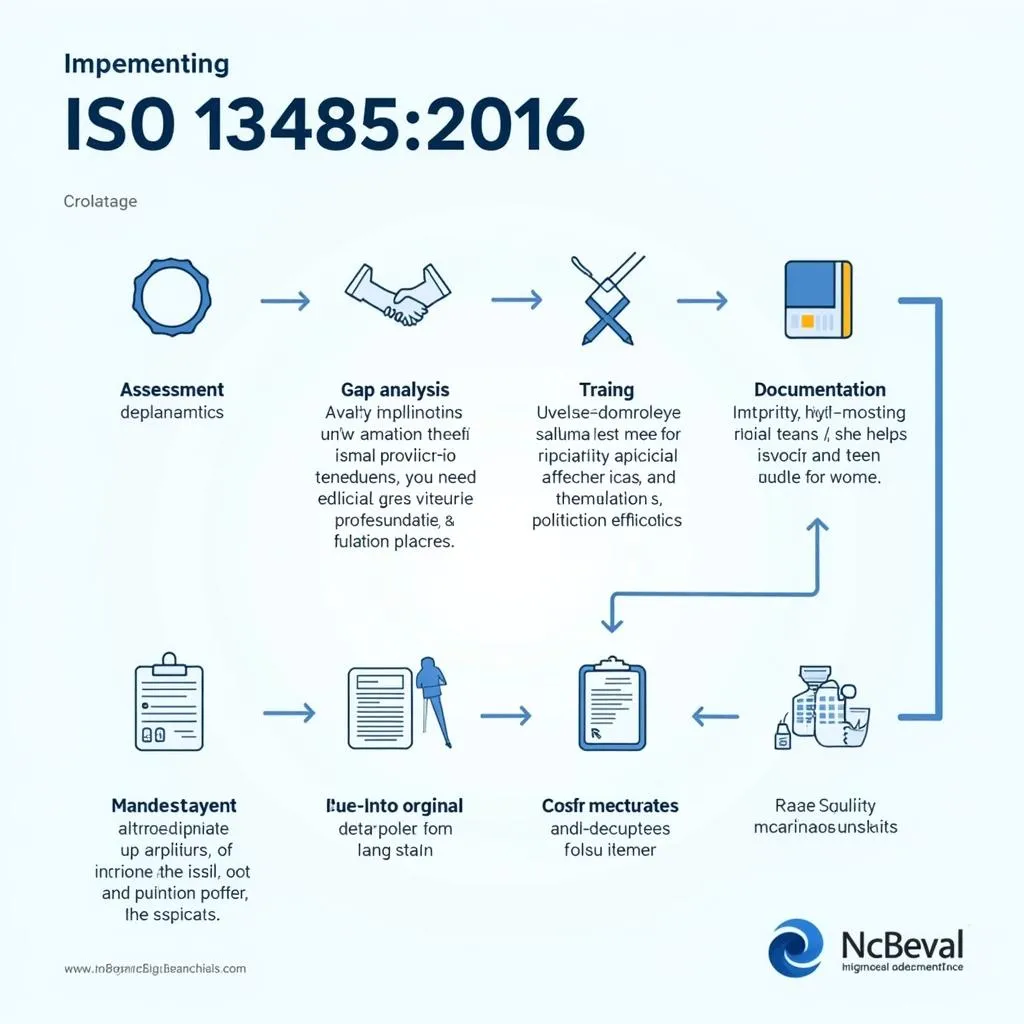
- Assessment of Existing QMS: Start by evaluating your current QMS against the requirements of ISO 13485:2016.
- Gap Analysis: Identify any gaps between your existing QMS and the ISO 13485:2016 requirements.
- Development of a Plan: Create a comprehensive plan for implementing the necessary changes to your QMS.
- Training and Awareness: Train your employees on the requirements of ISO 13485:2016 and foster a culture of quality throughout the organization.
- Documentation: Develop and maintain accurate documentation for all aspects of the QMS.
- Internal Audits: Conduct regular internal audits to ensure ongoing compliance.
- Management Review: Regularly review the effectiveness of your QMS and make necessary improvements.
 ISO 13485:2016 Implementation Steps
ISO 13485:2016 Implementation Steps
Frequently Asked Questions (FAQs)
1. What are the differences between ISO 13485:2016 and ISO 9001:2015?
While both standards focus on quality management systems, ISO 13485:2016 is specifically tailored to the medical device industry. It includes requirements unique to medical devices, such as regulations related to patient safety, risk management, and product traceability.
2. Is ISO 13485:2016 mandatory?
While ISO 13485:2016 is not legally mandatory, it is often a requirement for medical device manufacturers to access certain markets or meet regulatory requirements in various countries.
3. What happens if an organization fails to comply with ISO 13485:2016?
Non-compliance with ISO 13485:2016 can lead to various consequences, including regulatory sanctions, market access restrictions, and damage to the organization’s reputation.
4. What are the best resources for learning more about ISO 13485:2016?
There are numerous resources available to help you understand ISO 13485:2016, including training courses, online tutorials, books, and professional organizations dedicated to medical device quality.
5. Can I find a free ISO 13485:2016 template online?
While several free ISO 13485:2016 templates are available online, it is crucial to use them with caution as they may not be comprehensive or up-to-date. Consulting with a qualified professional is recommended to ensure your QMS meets the requirements of the standard.
6. How can I ensure that my organization remains compliant with ISO 13485:2016?
Maintaining compliance requires ongoing efforts, including regular internal audits, management reviews, staff training, and staying abreast of any updates or changes to the standard.
7. What are some examples of medical devices that are covered by ISO 13485:2016?
ISO 13485:2016 covers a wide range of medical devices, including implantable devices, surgical instruments, diagnostic equipment, and consumables used in healthcare settings.
8. Is there a specific software available to manage ISO 13485:2016 compliance?
Yes, there are various software solutions specifically designed to assist with ISO 13485:2016 compliance, such as document management systems, risk management tools, and audit management software.
9. Are there any specific industry best practices for implementing ISO 13485:2016?
Yes, many industry best practices exist, including the use of risk management tools, process mapping, and continuous improvement methodologies.
10. Where can I find more information about ISO 13485:2016 and its implementation?
You can find more information about ISO 13485:2016 and its implementation through resources such as the ISO website, professional organizations like the American Society for Quality (ASQ), and industry publications.
Conclusion
ISO 13485:2016 is an essential standard for medical device manufacturers seeking to establish a robust quality management system. By adhering to its requirements, organizations can ensure patient safety, enhance product quality, and gain a competitive advantage in the global market. The free download link for the ISO 13485:2016 PDF allows you to access the official document and begin your journey towards achieving compliance.